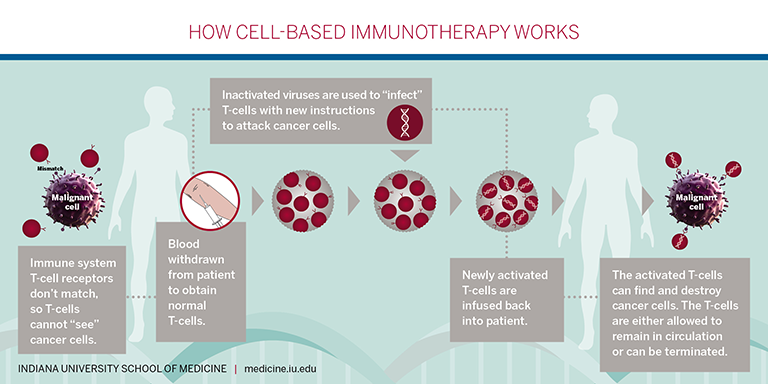
Currently, CAR T-cell therapy is approved by the FDA for:
- Adults with relapsed or refractory multiple myeloma who have completed at least four previous treatments.
- Adults with relapsed or refractory B-cell lymphomas, including diffuse large B-cell lymphoma, mantle cell lymphoma, and follicular lymphoma.
- Adults and children with relapsed or refractory acute lymphoblastic leukemia.
Clinical trials and FDA-approved treatments have proven CAR T-cell therapy is safe and effective. The results show that the enhanced T-cells can remain in the body long enough to eliminate cancer cells – and possibly prevent them from recurring.
Patients are usually admitted to the hospital for two to three weeks. After the CAR T-cells are infused, close monitoring from the care team ensures accurate testing of the immune system’s progress, as well as a quick response to any side effects that may occur.
The most common side effect of CAR T-cell therapy is called cytokine release syndrome, or CRS. It’s also known as a “cytokine storm.” It’s a consequence of CAR T-cells multiplying in the body, and about 70 percent to 90 percent of patients experience it.
CRS starts around the second or third day after the infusion, and symptoms are usually short-lived and reversible. It’s like having a severe case of the flu, with high fever, fatigue, and body aches. If CRS is severe and lasts longer than it should, the care team can administer an FDA-approved drug called Tocilizumab.
The other side effect is known as CRES, which stands for CAR T-cell-related encephalopathy syndrome. It typically starts around day five after the infusion. Patients can become confused and disoriented, and sometimes may not be able to speak at all for a few days. CRES can be upsetting for patients and their families, but it typically is completely reversible.