Studying peripheral neuropathy in Black women with breast cancer
The EAZ171 study is designed to help learn why Black women experience more neuropathy and which drugs are best at reducing it.
The EAZ171 study is designed to help learn why Black women experience more neuropathy and which drugs are best at reducing it.

Researchers at the IU Simon Comprehensive Cancer Center have discovered that Black patients with breast cancer who are treated with a chemotherapy called docetaxel experience less of a harmful side effect called peripheral neuropathy. Their findings represent an important shift in knowledge about a patient population who’ve historically been underrepresented in breast cancer research.

Recent research shows that patients of African ancestry who take chemotherapy drugs such as docetaxel or paclitaxel have a higher risk for neuropathy.
Symptoms of this side effect most often appear in the hands and feet and include:
These symptoms are collectively called neuropathy. Through this trial, we can identify the better treatment specific to women of African ancestry with breast cancer to reduce neuropathy.
Because of past wrongs done by researchers and a lack of trials focused on patients of African ancestry, Black patients are strikingly under-represented within clinical trials.
As a result, our knowledge of cancer treatments is based mostly on the information gathered from white patients. Our research shows that Black patients have a much higher risk of experiencing side effects from chemotherapy, especially neuropathy.
Neuropathy causes doctors to lower or even stop chemotherapy doses in their patients. In turn, breast cancer comes back (recurs) more often in Black patients than white patients, resulting in lower survival rates among Black patients.
Neuropathy is painful, can impact quality of life, and is sometimes permanent.
The American Society of Clinical Oncology considers neuropathy caused by chemotherapy to be one of the three most important survivorship issues impacting cancer patients.
If your treatment team decides chemotherapy with paclitaxel or docetaxel is right for you and you decide to take part in this study, you will:
After you finish your study treatment, your doctor will continue to follow you for up to five years and check with you for side effects. You will be asked to return six months after you join the study for another optional blood draw.
This study has two study groups. Both groups use FDA-approved treatments for your cancer.
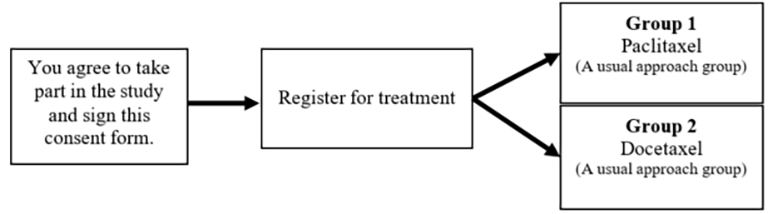
If you are in this group, you will get paclitaxel. You will receive this drug through a vein in the arm once a week for 12 weeks. There will be about 120 people in this group.
If you are in this group, you will get docetaxel. You will receive this drug through a vein in the arm once every three weeks for up to 18 weeks. There will be about 120 people in this group.
Talk with your doctor about which group is the right choice for you.
Clinical trial EAZ171 is being led by the ECOG-ACRIN Cancer Research Group (ECOG-ACRIN), which focuses its research on adults who have or are at risk of developing cancer.
ECOG-ACRIN receives funding for this trial from the National Cancer Institute, part of the National Institutes of Health.
Study chair Bryan Schneider, MD, discusses EAZ171, a clinical trial for Black women with breast cancer. Dr. Schneider hopes to improve treatment outcomes and decrease neuropathy, a painful side effect of some chemotherapy drugs.
This study is closed to accrual.